A Gaseous Mixture of O2 and N2
If a system contains a mixture of nitrogen oxygen and carbon dioxide gas then there exist only one phase because these gases form a homogeneous mixture and have the same. What is the partial pressure of oxygen in the mixture if the total pressure is 525 mmHg.

Solved A Gaseous Mixture Of O2 And N2 Contains 32 8 Nitrogen By Mass What Is The Partial Pressure Of Oxygen In The Mixture If The Total Pressure Is 805 Mmhg
A gaseous mixture of O2 and N2 contains 348 nitrogen by mass.

. Express you answer numerically in millimeters of mercury. Express you answer numerically in millimeters of mercury. Add your answer and earn points.
Up to 256 cash back A gaseous mixture of O2 and N2 contains 358 nitrogen by mass. P o 2 028 1. A gaseous mixture of O2 and N2 contains 358 nitrogen by mass.
A 100 L flask contains a mixture of methane and argon gasses at 25 degree C. 20 ml of a gaseous mixture of N2 and H2 gases is mixed with 8 ml O2 and the mixture is firedif the final volume becomes 13 ml the volume percent of N2 in the. 2912 28 4 x 1.
A gaseous mixture of O2 and N2 contains 408 nitrogen by mass. This will give you. 130 mmHg CO2 210 mmHg Ar and 182 mmHg O2.
P N 2 mole fraction of. Up to 256 cash back A gaseous mixture of O2 and N2 contains 398 of nitrogen by mass. X 1 028.
To calculate the pressure of the mixture we use ideal gas equation. P O 2 028 a t. P N 2 nN 2 P total nN 2 nO2 nCO2.
A gaseous mixture of O2 and N2 contains 308 nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 385 mmHg. According to mole concept.
The ratio of the mole numbers of N2 to O2 is 31. A gas mixture with a total pressure of 760 mmHg contains each of the following gases at the indicated partial pressures. A gaseous mixture contains O2 and N2 in the ratio of 1.
X 1 112 4. The exit mixture can be assumed to be in chemical equilibrium with CO2 CO O2 and N2 present. Calculate the total kinetic energy of gaseous mixture.
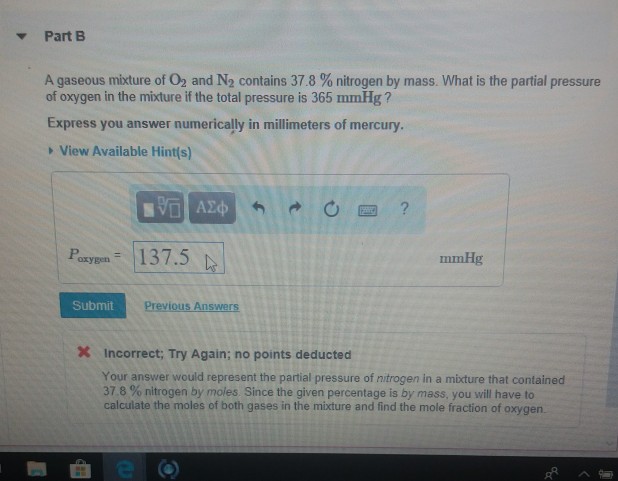
A gaseous mixture of O2 and N2 contains 398 of nitrogen by mass. A gas mixture consists of O2 and N2. What is the partial pressure of oxygen in the mixture if the total pressure is 365 mmHg.
A gas mixture of 1 kmol carbon monoxide 1 kmol nitrogen and 1 kmol oxygen at 25C 150 kPa is. A gaseous mixture of O2 and N2 contains 308 nitrogen by mass. Express you answer numerically in millimeters of mercury.
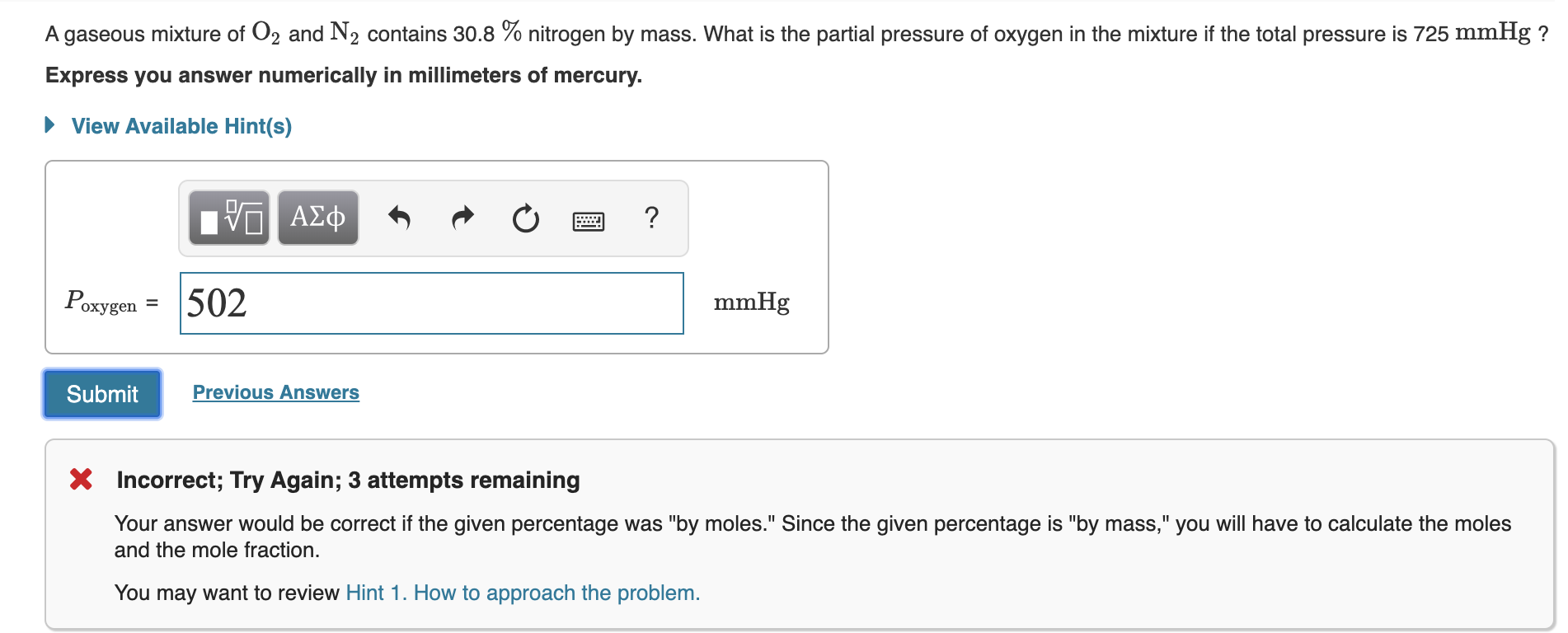
Shaelycole9389 is waiting for your help. What is the partial pressure of oxygen in the mixture if the total pressure is 725 mmHg. A gaseous mixture consist of 691 grams of N2 471 grams of O2 and 295 grams of He.
In a system phase is defined as the portion of the system which has different physical properties and can be separated by many mechanical processes. Whah is the partial pressure of oxygen in the mixture if the totally pressure is 766 mmHg. What volume does this mixture occupy at 28 degree celcius and 105 atm pressure.
P total P N 2 P O2 P CO2. A gaseous mixture of O2 and N2 contains 368 nitrogen by mass. Equal moles of O2 and N2 are present in a gaseous mixture.
If the total pressure of the mixture is found to be 760 mm of Hg then partial pressure of the oxygen O2 in the mixture is 760 mm Hg 380 mm Hg 480 mm Hg 253 mm Hg. A cyclopropane-oxygen mixture is used as an anesthetic. Express you answer numerically in millimeters of mercury.
P o 2 x 1 P t o t a l. What is the partial pressure of oxygen in the mixture if the total pressure is 565 mmHg. Heated in a constant pressure process.
Question A gaseous mixture of O2 and N2 contains 338nitrogen by. A gaseous mixture of O2 and N2 contains 308 nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 405mmhg.
The mass of argon present is 228 g and the mole fraction of methane in the mixture is 0650. What is the partial pressure of oxygen in the mixture if the total pressure is 765 mmHg. This mixture is heated during a steady-flow process from 180 to 210 K at a constant pressure of 8 MPa.
Putting values in above equation we get. Given mass of nitrogen gas 035 g. So 5600 mL of volume will be occupied by of oxygen gas.
224 L of 22400 mL of volume is occupied by 1 mole of a gas. See the answer See the answer done loading. The system has one phase.
Determine the heat transfer during this process per mole of the mixture using a the ideal-gas approximation and b Kays rule. 4 x 1 112. What is the partial pressure of oxygen in the mixture if the total pressure is 665 mmHg.
A gaseous mixture of O 2 and N 2 contains 338 nitrogen by mass. As total pressure of the system is 1 atm and we also know the mole fraction of oxygen therefore we will used daltons law of partial pressure. Now to get the partial pressure of lets say nitrogen gas you would use equation 1 RT V P total nN 2 nO2 nCO2.
Molar mass of nitrogen gas 28 gmol. The ratio of their number of molecules is.

Solved A Gaseous Mixture Of O2 H2 And N2 Has A Total Pressure O Chegg Com
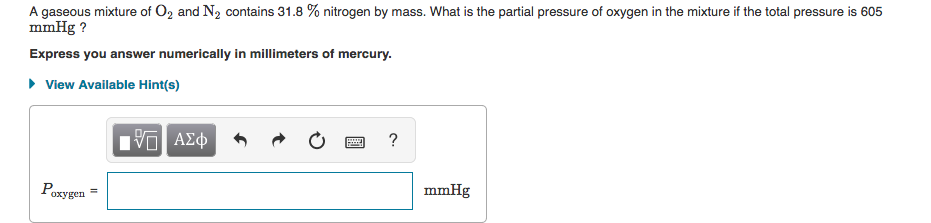
Solved A Gaseous Mixture Of O2 And N2 Contains 31 8 Chegg Com

Solved A Gas Mixture Contains Each Gas At The Indicated Partial Pressure Begin Array Ll Mathrm N 2 217 Text Torr Mathrm O 2 106 Text Torr Mathrm He 248 Text

Solved Open Ended Question A Gaseous Mixture Of O2 And N2 Chegg Com
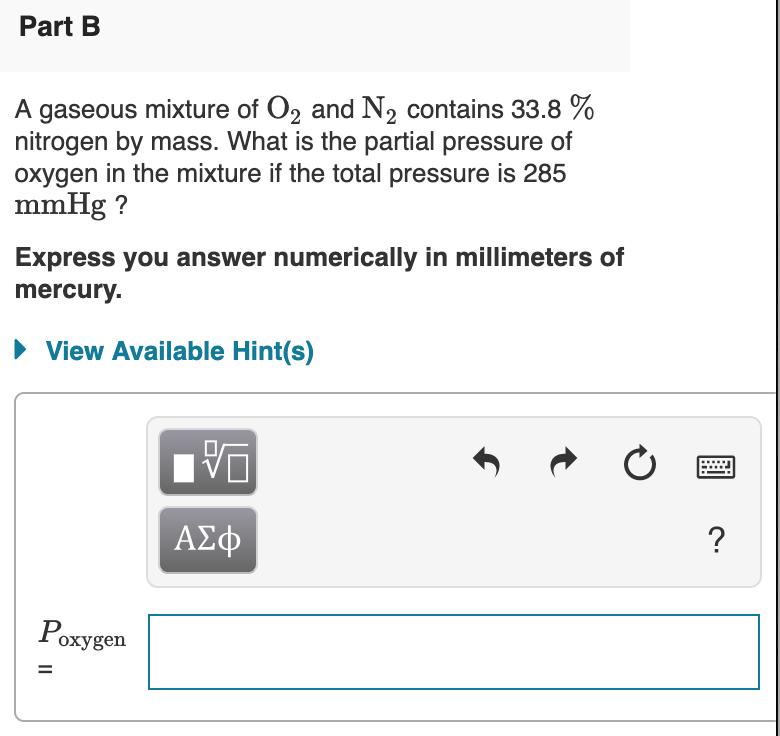
Solved Part B A Gaseous Mixture Of O2 And N2 Contains 33 8 Chegg Com
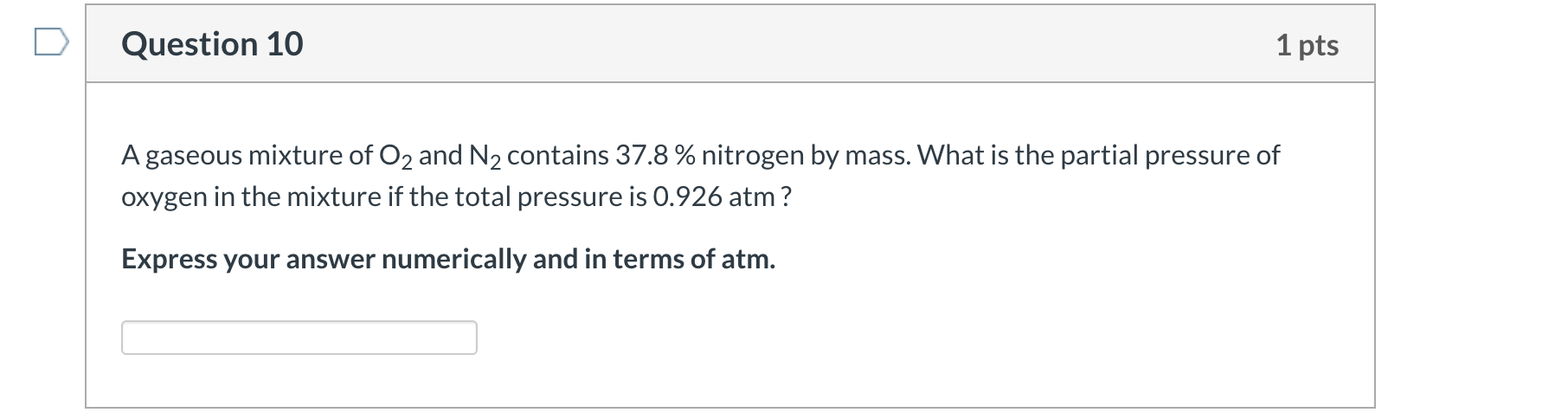
Solved Question 10 1 Pts A Gaseous Mixture Of O2 And N2 Chegg Com

A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 4 By Weight Therefore

A Gases Mixture Contains Oxygen And Nitrogen In The Ratio 1 4 By Weight Therefore The Rati Youtube
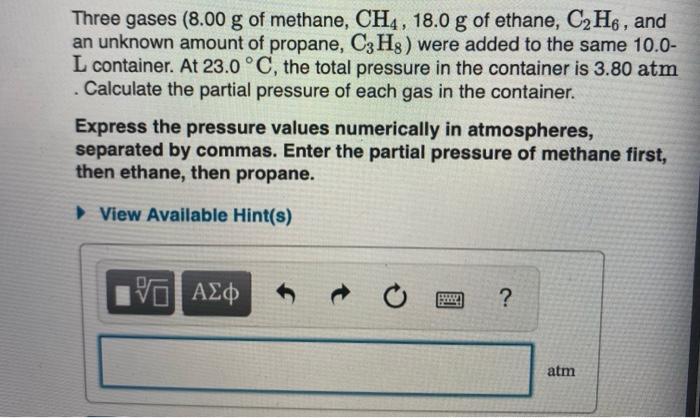
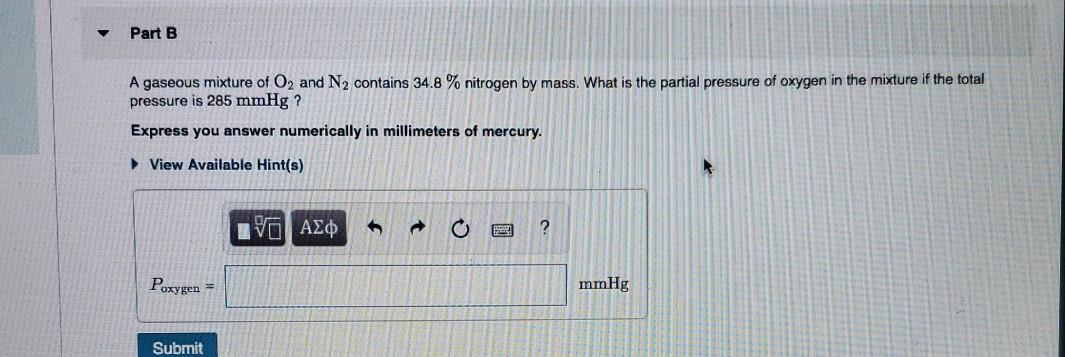
Solved A Gaseous Mixture Of O2 And N2 Contains 34 8 Chegg Com
Answered A Gaseous Mixture Of O2 And N2 Contains Bartleby

Solved A Gaseous Mixture Of O2 Molecular Mass 32 U And N2 Chegg Com
Solved Part B A Gaseous Mixture Of O2 And N2 Contains 37 8 Chegg Com
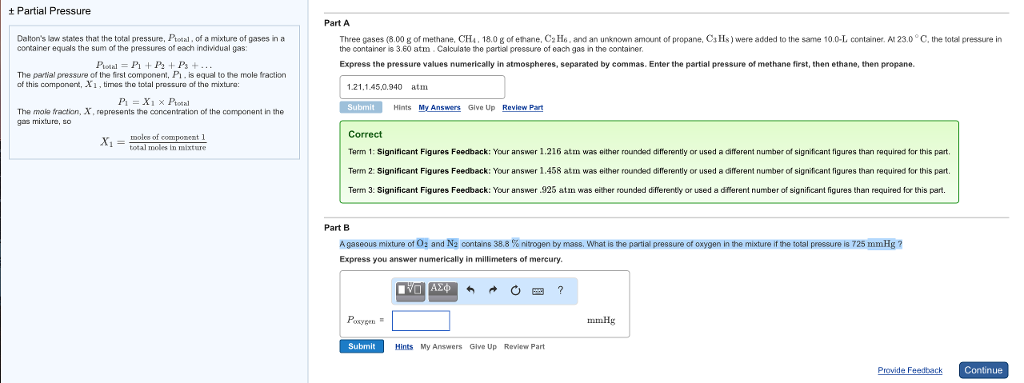
Solved A Gaseous Mixture Of O2 And N2 Contains 38 8 Chegg Com

A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 4 By Weight Therefore

A Gaseous Mixture Of O2 And N2 Contains 37 8 Nitrogen By Mass What Is The Partial Pressure Of Brainly Com

40ml Gaseous Mixture Of Co Ch 4 And Ne Was Exploded With 10ml Of Oxygen Youtube
Solved Part B A Gaseous Mixture Of O2 And N2 Contains 34 8 Chegg Com
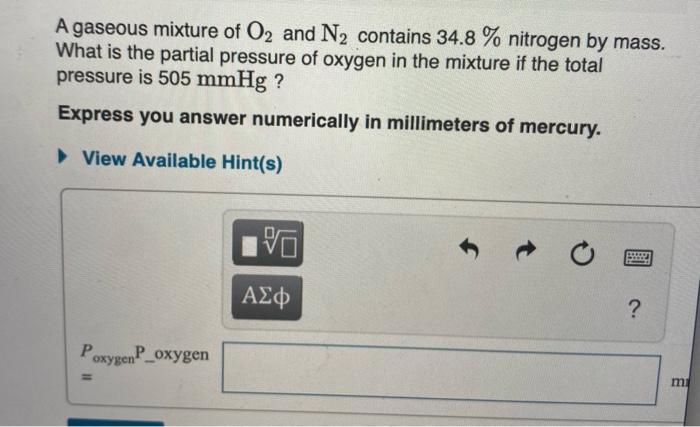
Solved A Gaseous Mixture Of O2 And N2 Contains 34 8 Chegg Com
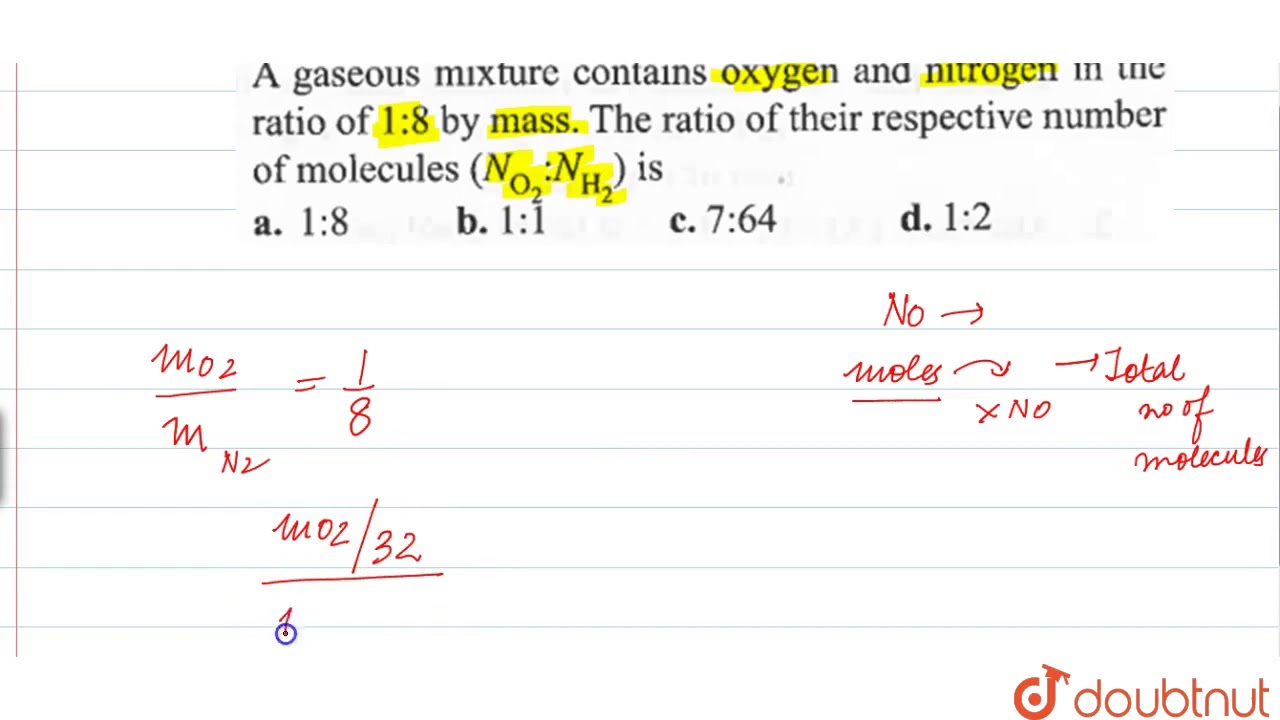
A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 8 By Mass The Ratio Youtube
Comments
Post a Comment